Química
Boyle's Law
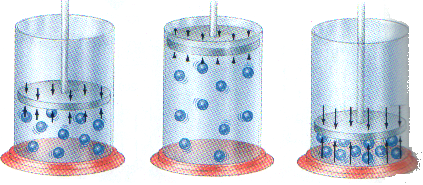
BOYLE'S LAW
OBJECTIVES
-
Describe in qualitative terms the effects of changes in pressure and volume on contained gases.
-
Calculate pressure or volume from the pressure - volume relationship of a contained gas at constant temperature.
-
PROBLEM
-
Variation between the pressure and volume.
-
HYPOTHESIS
-
The pressure and volume vary inversely proportional.
-
MATERIALS
-
A syringe
-
A graphic calculator
-
CBL
-
A pressure sensor.
-
PROCEDURE
-
Turn on CBL and the graphic calculator.
-
To program Chembio.
-
To put prove, channel, calibration, used stored in that order.
-
To press Triger in CBL after each measure bye the pressure sensor.
-
When you finish all measures to put in the graphic calculator “No repeat”, first curve, and Power L1, L2.
-
DATA
-
The equation for calculate the heat released is:
-
P = pressure
-
V = volume
-
OBSERVATIONS
-
ANALYSIS
-
CONCLUSIONS
-
When the pressure goes up, the volume goes down.
-
The relationship between pressure and volume is inversely proportional.
-
The product of volume and pressure at any two sets of conditions is always constant at a given temperature.
-
Pressure increased when volume decreased because the gas molecules have minor space and the container received most fight by the molecules inside its.
-
The collision of the particles in a gas with the walls of the container is gas pressure.
-
The pressure in the container increases in proportion to the number of gas particles inside its.
-
The gases' constituent corpuscles stand still always they are in contact whit others.
-
The gases' constituent corpuscles can compress them, this one explains their capacity for to decreased the volume when a exterior pressure affects them.
-
The volume of a container easily accommodates whichever number of particles.
-
The mechanics process in a laboratory make, sometimes, we obtain wrong answers.
| P1V1 = P2V2 |
Table Nº1
| Volume (L) | Pressure (Kpa) |
| 2.5 | 179.87 |
| 5.0 | 101.18 |
| 7.5 | 72.45 |
| 10.0 | 56.21 |
| 12.5 | 46.22 |
| 15.0 | 38.72 |
| 17.5 | 33.73 |
Descargar
| Enviado por: | El remitente no desea revelar su nombre |
| Idioma: | inglés |
| País: | Estados Unidos |
